Non-Cultured Samples
The following is a short guide to the types of non-cultured samples analyzed by Fiberquant. It is not intended as an exhaustive sampling guide. Please refer to the following for sampling information: Bioaerosols Assessment and Control", American Conference of Government Industrial Hygienists, 1999 (opens in a new tab). Sampling materials, such as filter cassettes, microscope slides, Ziploc bags, and tape may be obtained from Fiberquant.
Analysis of Non-Cultured Fungi Samples
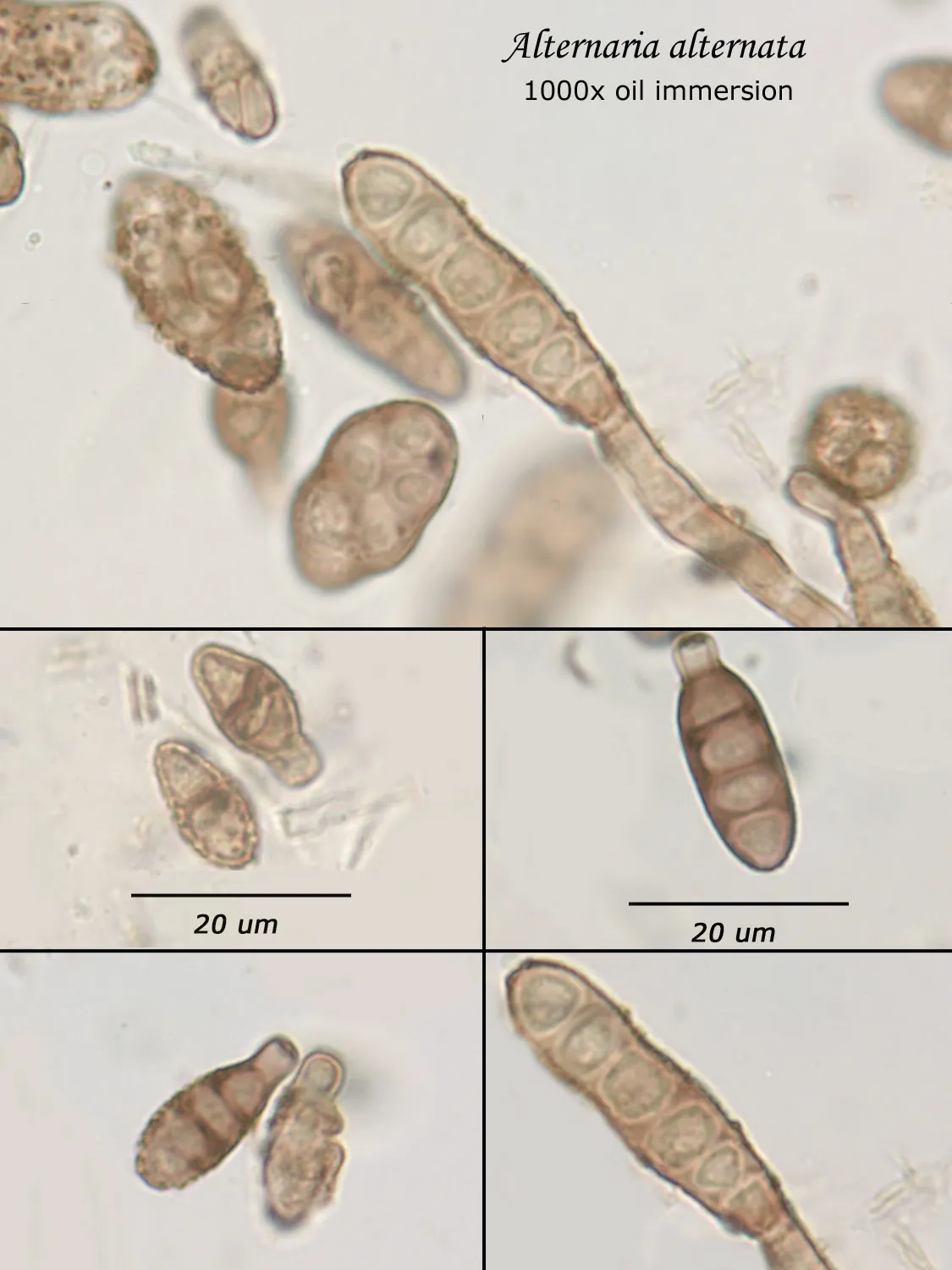
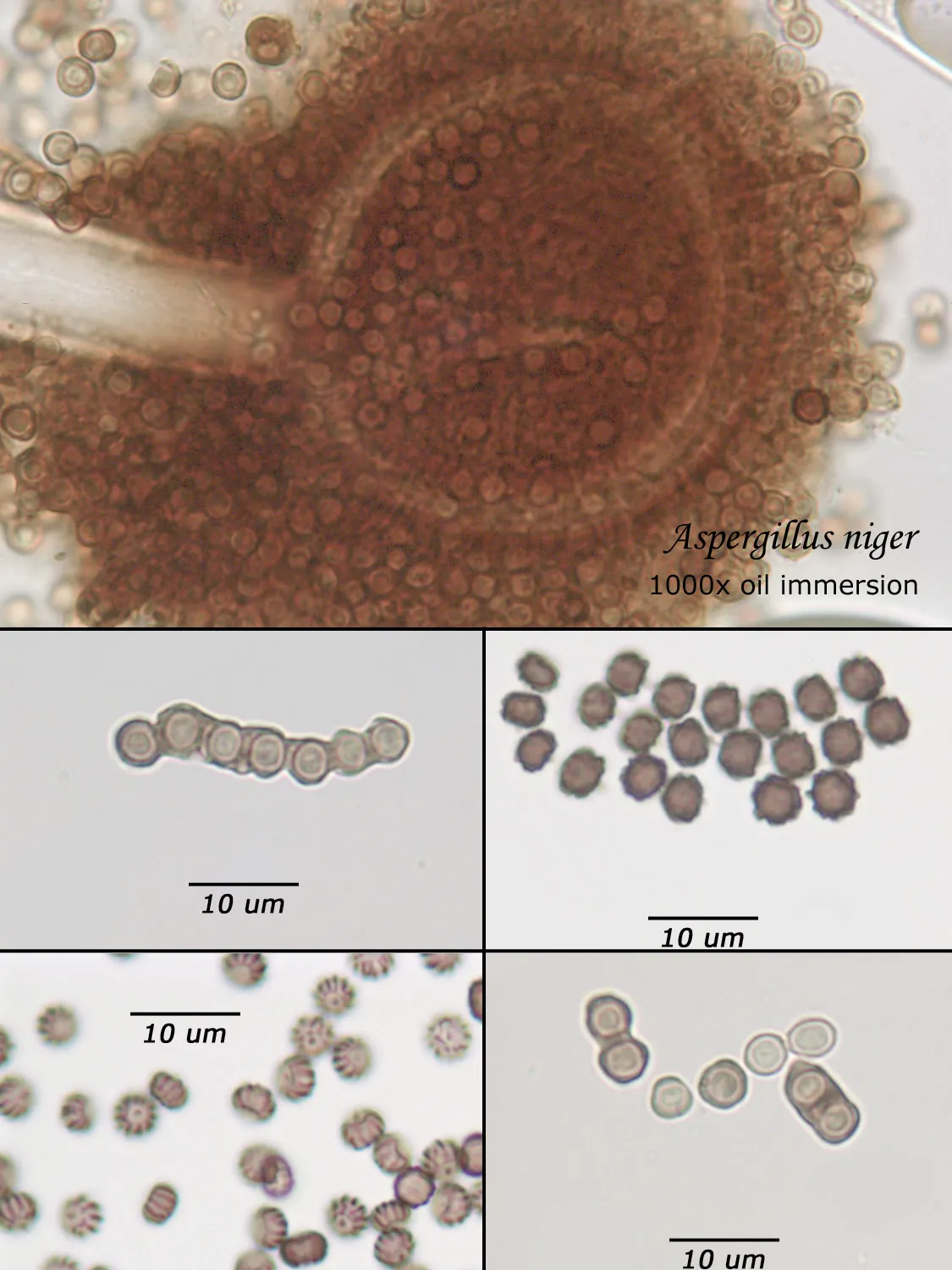
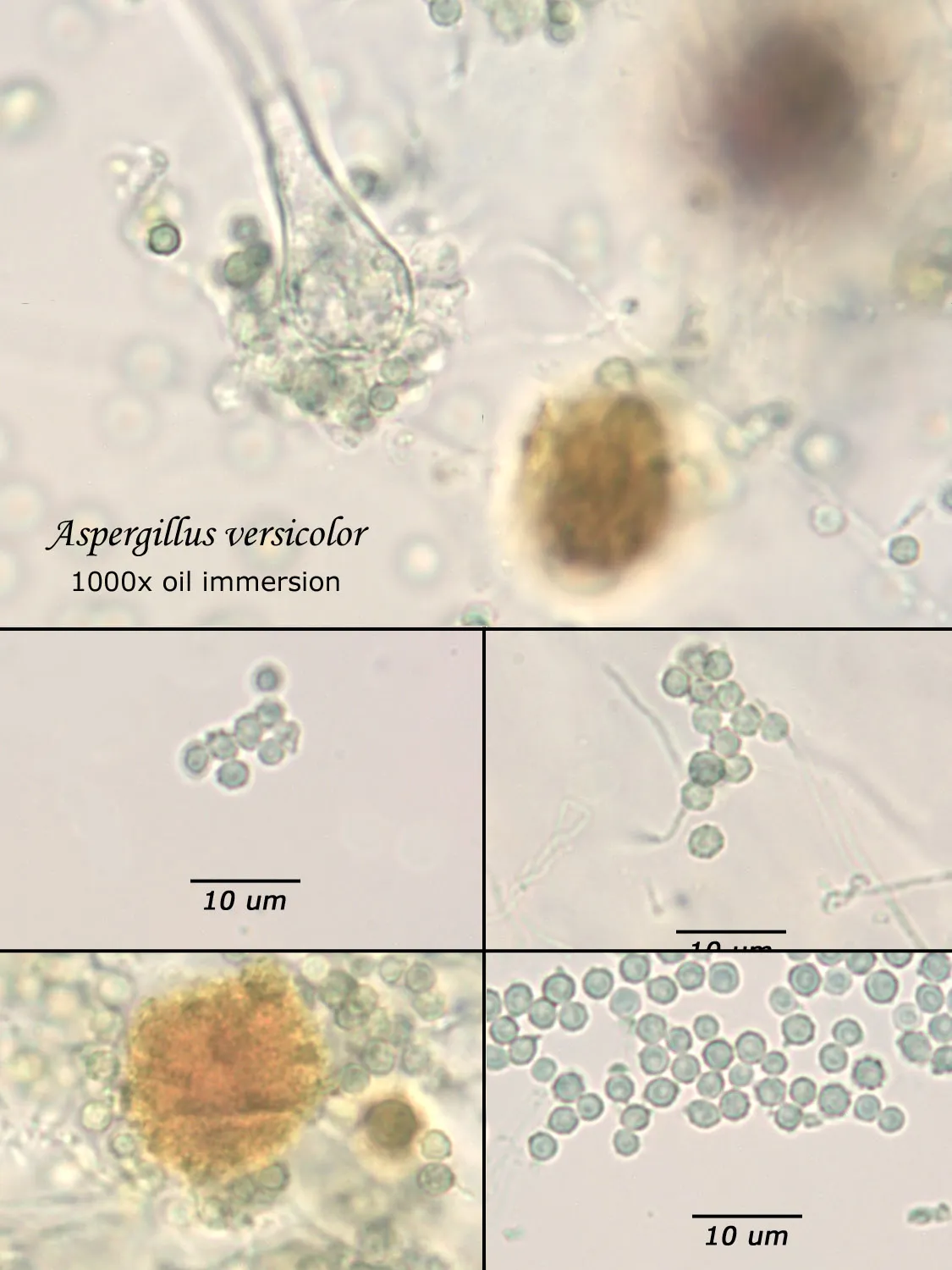
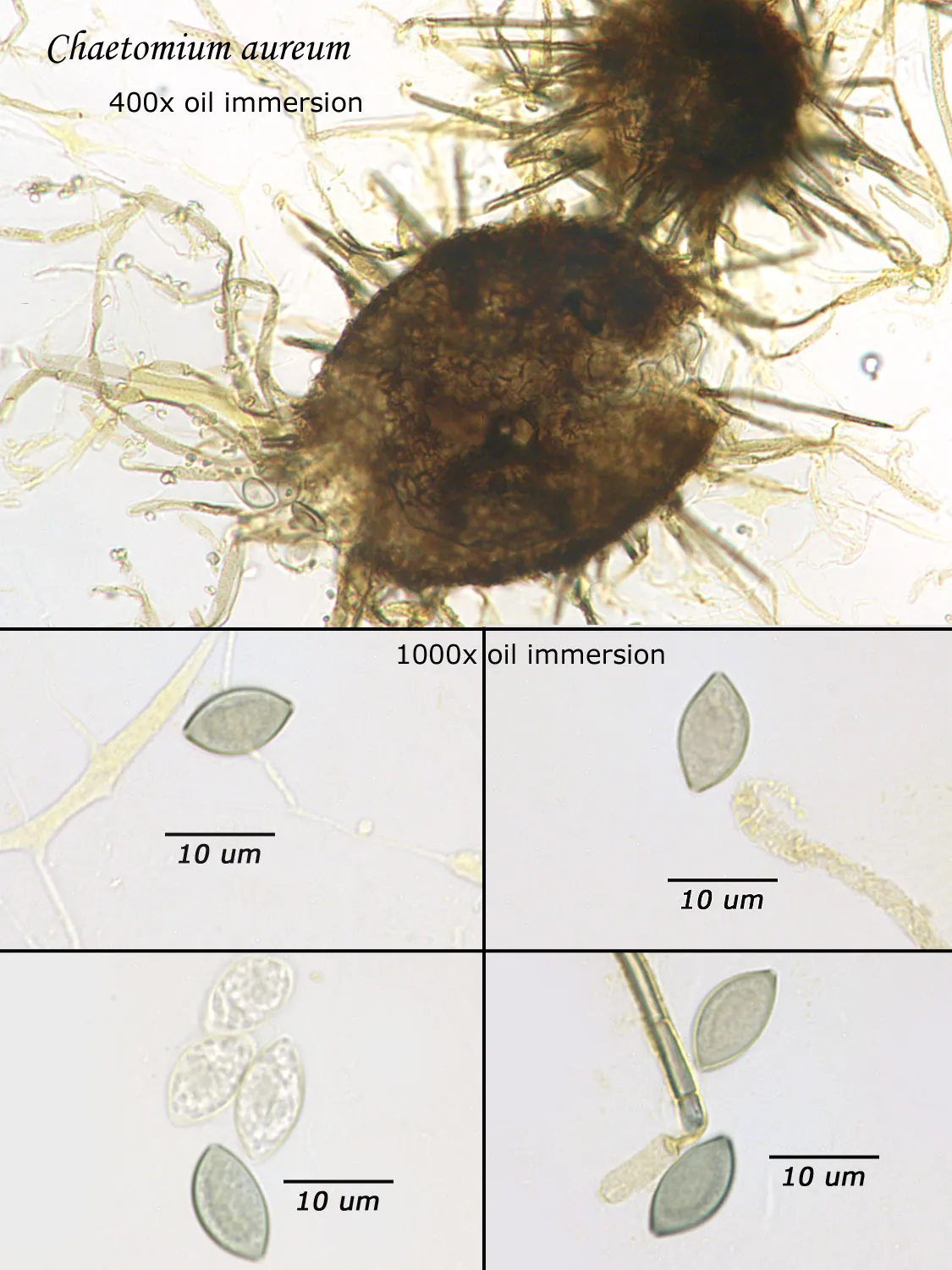
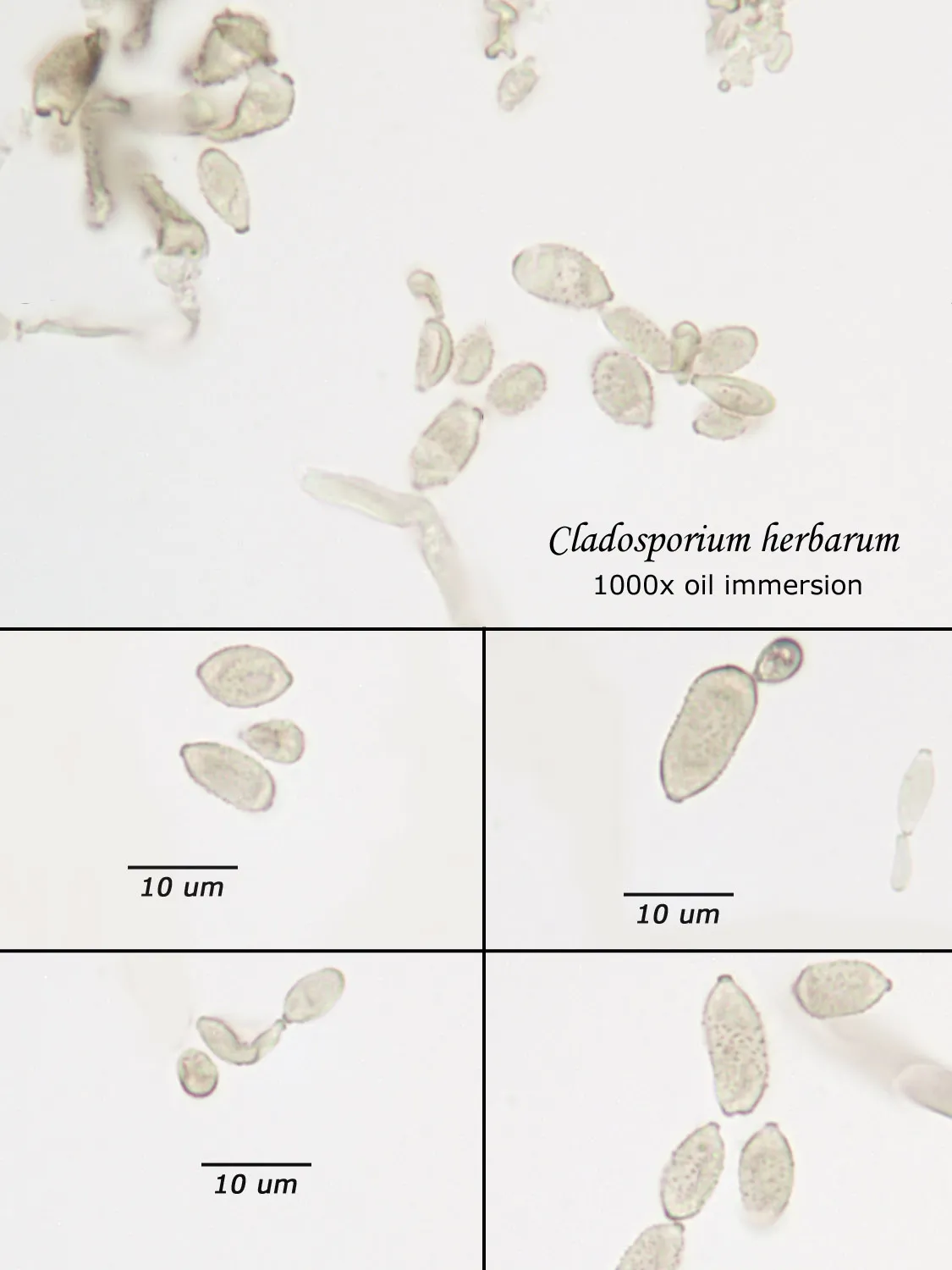
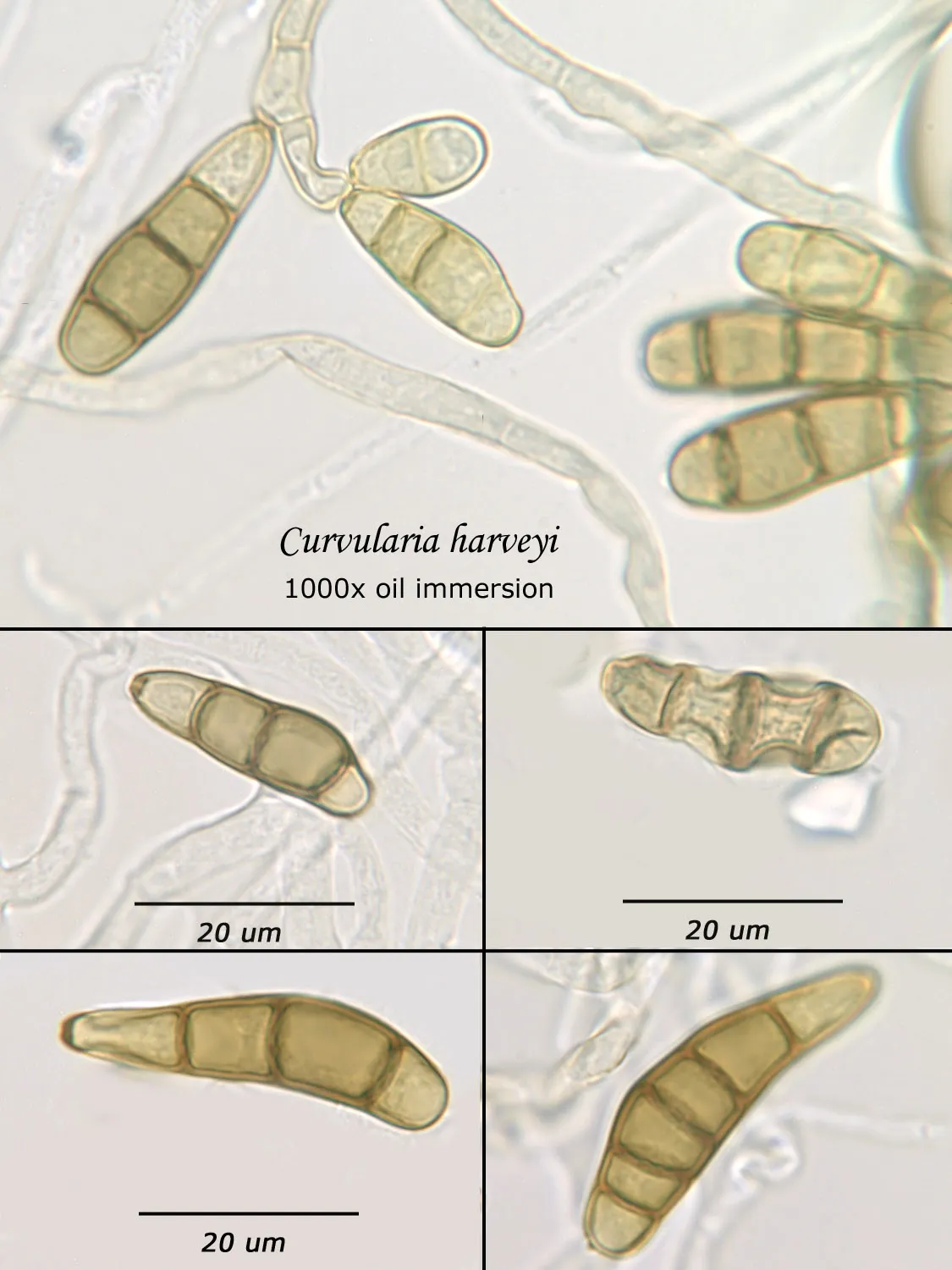
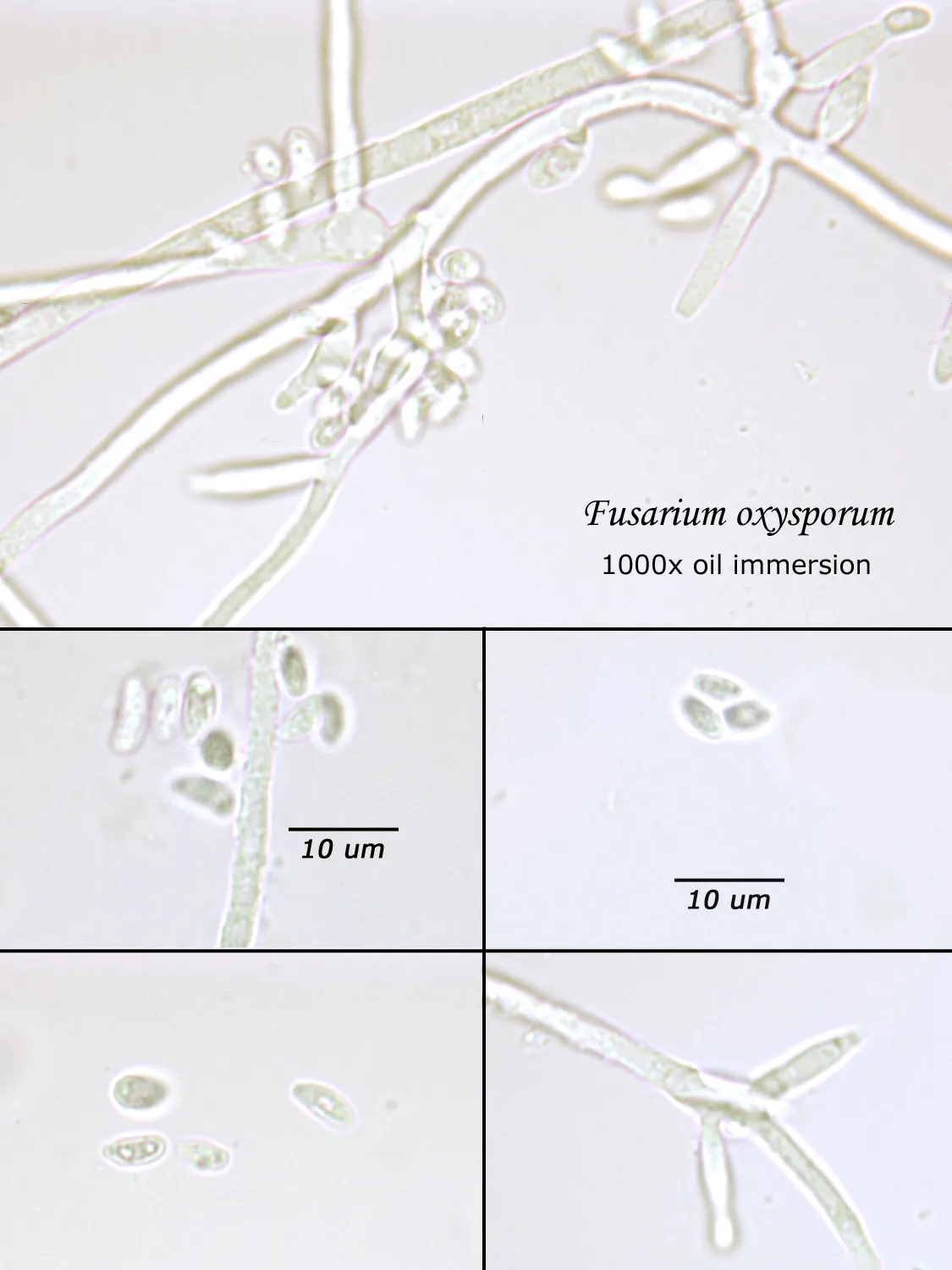
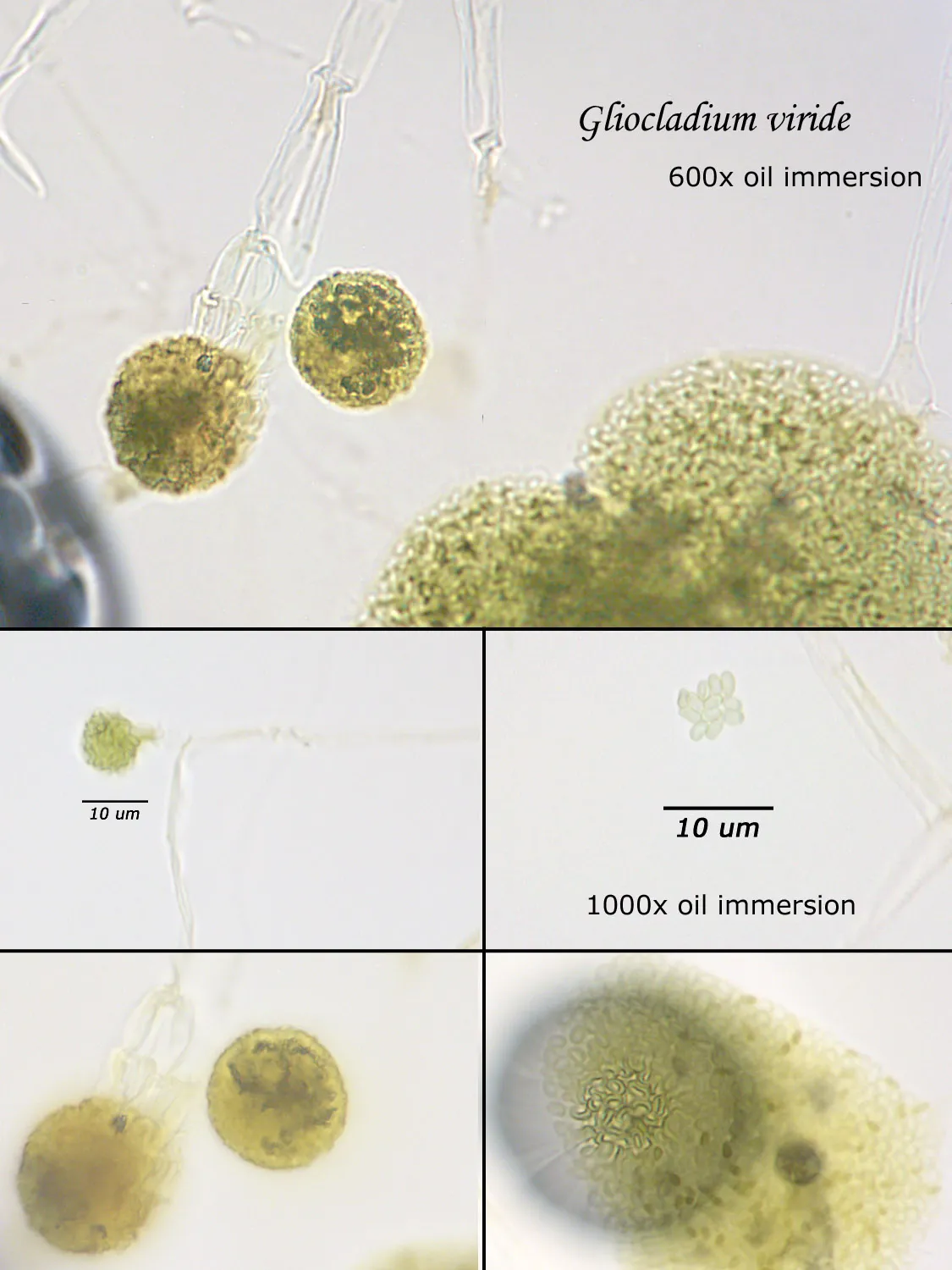
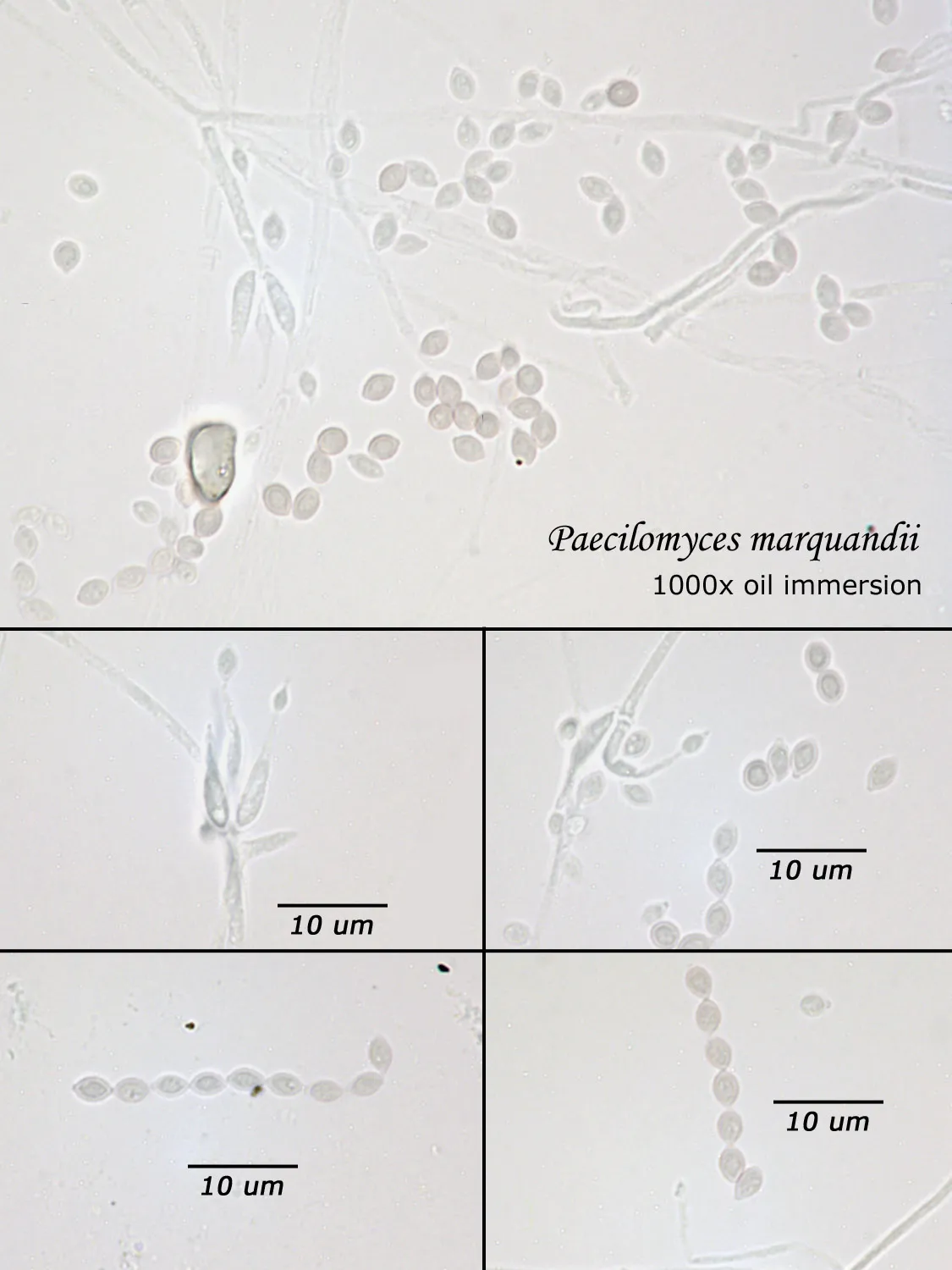
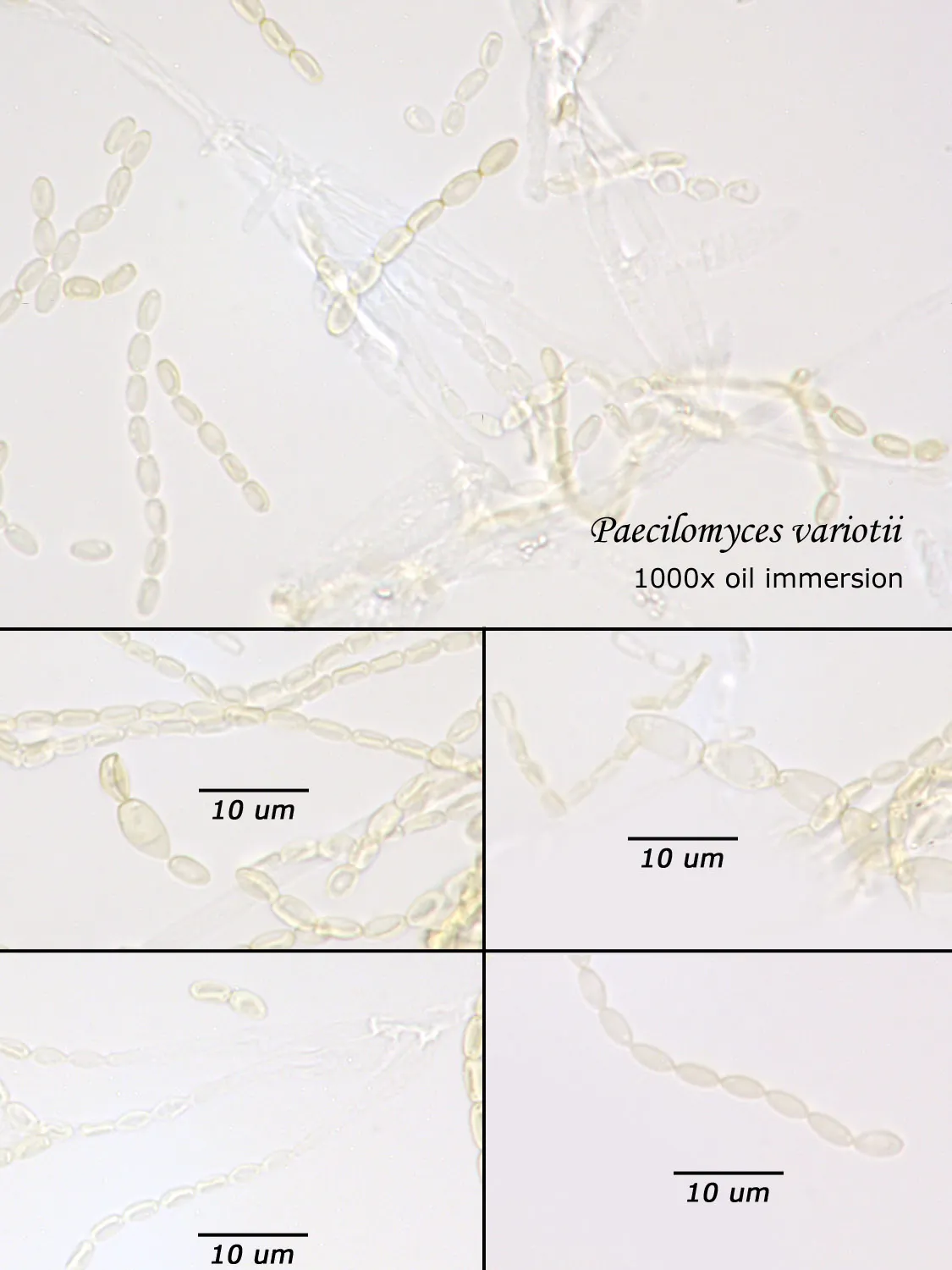
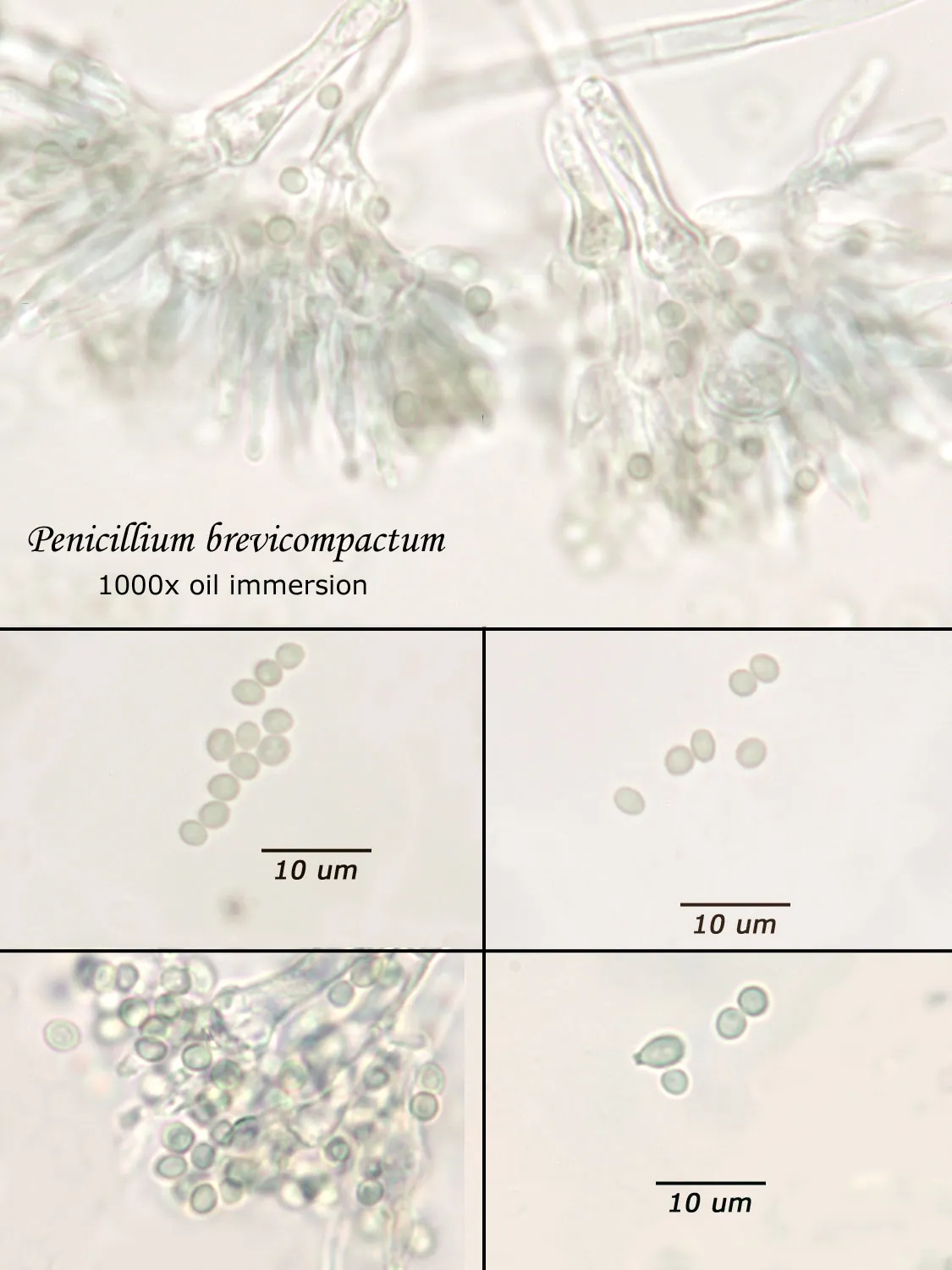
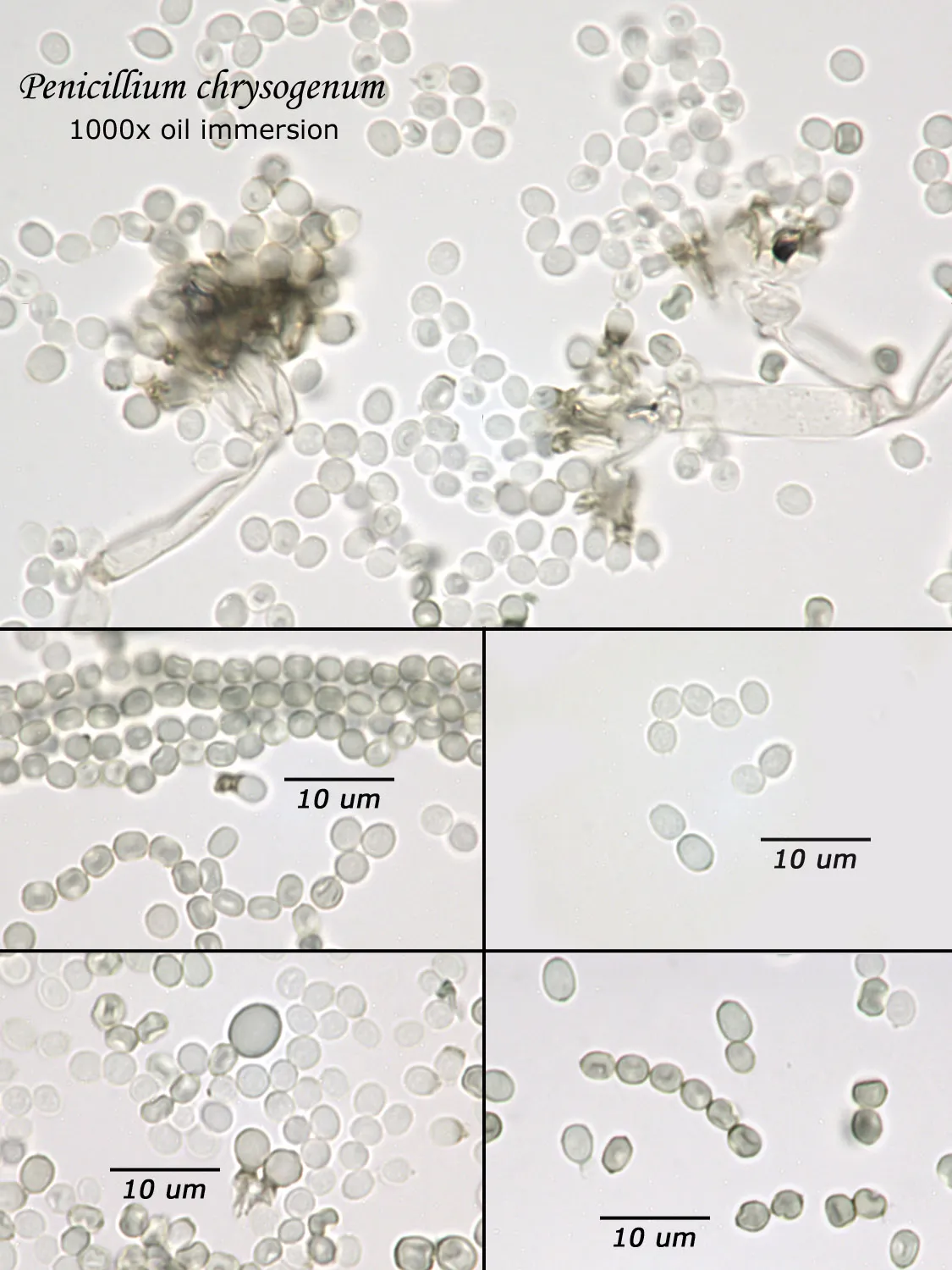
As opposed to cultured-type (sometimes called viable) analysis, in which an agar-containing pitri dish is used to grow colonies, non-cultured-type (sometimes called non-viable) analysis involves direct analysis of the fungal material present in the sample. The material may be growing on a substrate, captured on tape, or captured by an air sampling device. In order to analyze such samples, the sample or sub-samples are mounted on a microscope slide(s) to be examined at 200x-1000x (magnification depends on the type of sample). Spores and other fungal structures are identified to the genus level, if possible, by optical characteristics (e.g., spore shape, size, color, hypha morphology). The spores identified or counted may or may not be able to germinate (thus the name non-viable)
Interpretation of non-cultured sample data is complicated by two factors: 1) Usually, identification must be made from a spore or spores alone. In some cases, this is enough information to identify the genus (e.g., Stachybotrys). In other cases, spores from different genera look so similar that they cannot be distinguished (e.g., Penicillium and Aspergillus); therefore, these must be reported in a combined category (e.g., Penicillium/Aspergillus). 2) Spores are not the only fungal material observed in samples. Mycelial fragments occur unassociated with spores, and cannot be further identified except as mycelial fragments. In spore trap samples, the mycelial fragment count does not contribute to the total spore count. In bulk samples, mycelia that cannot be associated with some spore type are also reported separately, but, in this case, contribute to the total % fungus.
Bulk Sample
A bulk sample is a piece of building material or a tape lift taken from a building material. The purpose of this type of sample is to identify the mold. To sample, collect what appears to be one kind of mold. Many colonizations consist of multiple species. For a bulk sample, merely collect part of the material and place it in a Ziploc bag. For a tape lift sample, touch the middle of a 3” x 0.75” piece of tape (such as 3M Crystal Clear) to the colony. The deposit should be heavy enough to be easily seen on the tape. Adhere the tape to a clean glass slide. The middle may not adhere due to the deposit, but the ends will stick. In the lab, some of the bulk material will be mounted on a microscope slide, if not already, and the resulting spore types identified, as above. The results will be semi-quantitative: the identification of the type(s) of fungi observed and the estimated percentages of each. Swab samples may be used as an alternative to bulk and tape lift smaples.
Spore Trap Samples
Spores (and any other debris) are trapped as an air sample impinges a sticky surface. Typical volumes are 75-150 L, corresponding to 5-10 minutes of sampling. Sample volumes from areas expected to be spore-laden (e.g., affected rooms, moist climates) should be limited to 75 L. Samples of typical outdoor Arizona air should be 75-150 L. Samples from wall cavities are not recommended. In the lab, the cassette will be disassembled, and the inner cover slip-like piece that holds the tacky surface is removed and mounted on a glass slide. A drop light stain is added to emphasize colorless spores and to exclude air from the mount. Spores are counted as the thin strip of debris is scanned back and forth. Nominally, 30 such passes across the strip are made. Results will be (for each category of spore and total) raw counts, % of each category of spore, and counts/m3 of air.
Tape Lift (Analyzed per Area)
This type of sample might be used to check for contamination. In this case, the tape is touched once only to the surface (preferably a smooth surface, although fabrics have been tested like this also). The tape lift must be put on a clean slide in the field, since further manipulation can only invalidate the sample. In the lab, a certain area of the tape will be analyzed, nominally 50 fields of view. The results will be in spores/cm2 of tape.
Spore Trap Counting Methods
As of the current date, there is only one published consensus method (ASTM D7391) for counting spore trap samples. The ASTM method allows for some variation in counting techniques, which can introduce inter-laboratory variation.
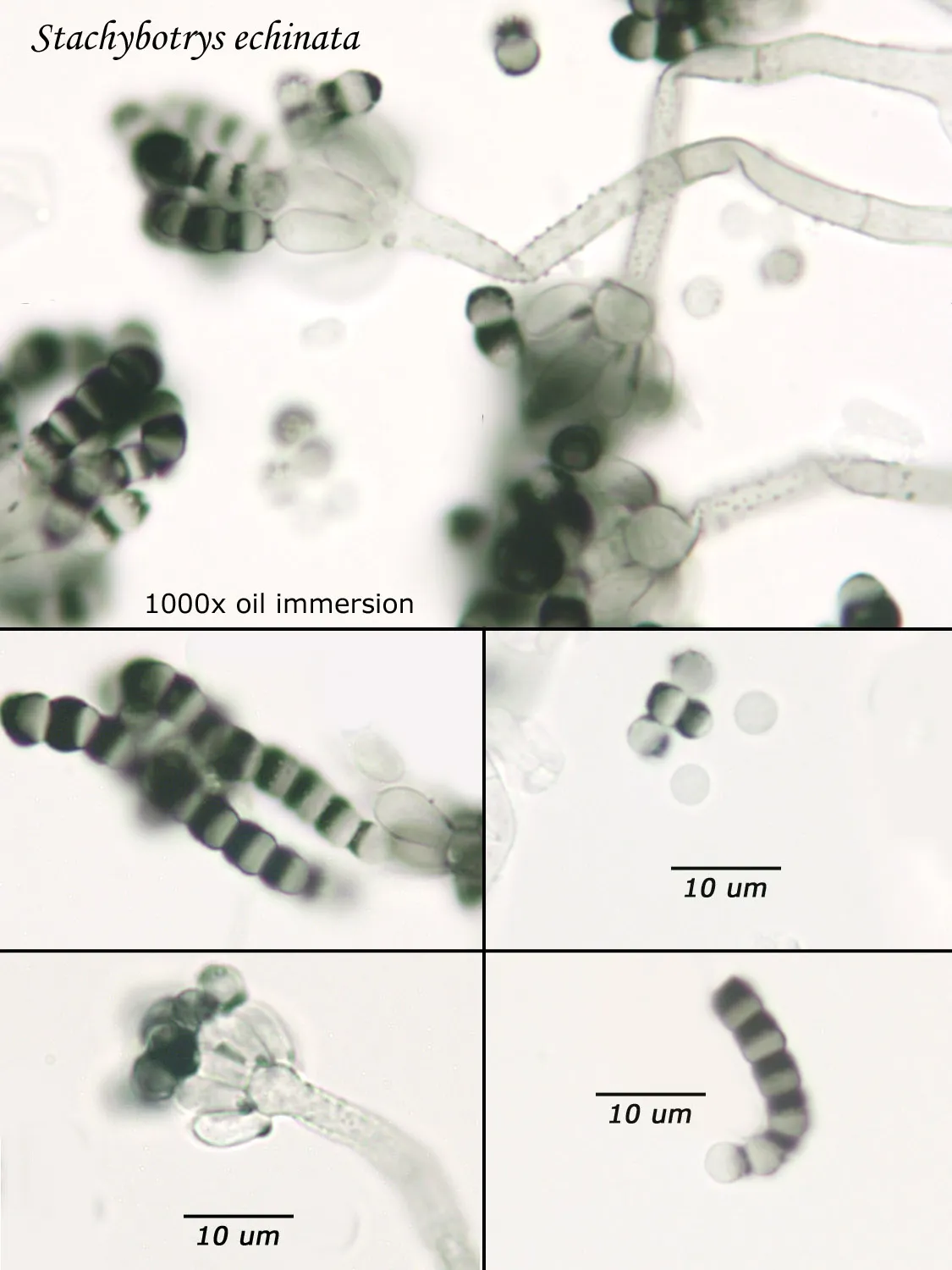
The Spore Trap Problem
Spores may be as small as 2um diameter or as long as 30 um. To see detail on a 2 um particle (e.g., smooth or rough, point of attachment, etc.) that may be required to distinguish spore types, the highest resolution available on an optical microscope is required, normally 1000x magnification using oil immersion. Ideally, the entire deposit would be examined at this magnification. However, the cost for such an analysis would be prohibitive. One compromise which can be made is to count a portion (20-25%) of the deposit. This has the advantage that any spores observed may be identified at the best resolution, but the disadvantage that clumps or strings of spores inhomogeneously distributed over the deposit will cause this type of analysis to be less reproducible than an analysis which counts the entire deposit. A different compromise which can be made is to count the entire deposit, but use a lower magnification (400-600x) which allows the analysis to be completed in an economically feasible amount of time. The advantage to this compromise is that its reproducibility should be high (depending on the skill of the analyst). Its disadvantage is that certain spore types which may be distinguished at 1000x may not be distinguishable at 400-600x. Another complicating factor is that 40-60x objective lenses (normally yielding 400-600x) are usually dry or air immersion in use, whereas the resolution desired at 1000x can only be achieved using a 100x oil immersion lens (100x dry lenses exist, but do not produce any better resolution than a dry 60x lens). Therefore, if one is scanning using a dry 60x lens, one cannot very well change to 100x oil, then go back to dry conditions. Once oil is on the slide it is difficult to clean off.
Fiberquant Analytical Services Approach
Recognizing that oil immersion is a requirement to the best resolution and best spore differentiation, Fiberquant uses oil immersion objectives for spore trap analysis. Also recognizing that medium magnification may have advantages in spore trap analysis, we have purchased for each microscope a 50-60x oil immersion objective as well as the usual 100x objective, allowing the analyst to readily change back and forth from 500-600x to 1000x, all in oil immersion mode, as required by the demands of the analysis.
In all spore counts, the interpretation of spore trap sample data is complicated by two factors: 1) Usually, identification must be made from a spore or spores alone. In some cases, this is enough information to identify the genus (e.g., Stachybotrys). In other cases, spores from different genera look so similar that they cannot be distinguished (e.g., Penicillium and Aspergillus); therefore, these must be reported in a combined category (e.g., Penicillium/Aspergillus-like). 2) Spores are not the only fungal material observed in samples. Mycelial fragments occur unassociated with spores, and cannot be further identified except as mycelial fragments. In spore trap samples, the mycelial fragment count does not contribute to the total spore count and are reported separately.
Spore Trap Analysis
This method uses both medium and high magnifications. A scan of the entire deposit is performed at 500-600x oil immersion, during which large spores, pollen, fragments, and clumps or clusters of spores are counted. Then, 30 passes across the deposit are performed, during which the small spores not in clumps or clusters are counted. The results from the two counts are mathematically combined into one result for each spore type. For a spore type expected to be reliably counted during the full scan, its spores/sample result is a count, reported to 1 spore, and its analytical sensitivity is 13 spores/m3 for a 75L sample. For a spore type expected to have counts at 1000x, its spores/sample result is a calculated value, reported to two significant figures, and its analytical sensitivity is 65-71 spores/m3 for a 75L sample.